Video About Medical Device Injection Mold
——
Description of Medical Device Injection Mold
——
KRMOLD specializes in the research, development, and manufacturing of medical device injection molds, providing professional solutions for a wide range of plastic medical device products. These products include disposable syringes, plastic test tubes, cell culture dishes, culture flasks, applicators, pipette tips, atomizer cans, and respirators. KRMOLD medical device plastic injection molds offer high quality, high precision, excellent light transmittance, and a dent-free finish. KRMOLD provides customers with complete plastic medical device injection molding solutions, including product design and development, mold design, mold process analysis, medical device injection mold development, a full line of equipment, after-sales installation, and training services.

Features of Medical Device Injection Mold
——
1) Widely applicable medical device injection molds KRMOLD medical device plastic injection molds can be used across various medical applications to manufacture common medical devices such as syringes, inhalers, medical trays, catheters, surgical tools, and components for blood glucose meters and blood pressure monitors. Single-use or reusable elements in the medical field can be plastic parts made with KRMOLD medical device plastic injection molds.
2) High-precision medical device injection molds To guarantee net-shape components made by KRMOLD engineers achieve exceedingly repeatable tolerances as low as +/- 0.001 inches or less using precision-machined steel molds. KRMOLD medical device injection molds hardly call for any additional processing. This makes it possible to preserve difficult geometries at high production levels within narrow tolerances.
3) High-volume medical device plastic injection molds KRMOLD medical device injection molds can produce thousands of parts per hour. Increased automation reduces labor costs, while low mold scrap rates and minimal secondary operations improve cost efficiency. The purchase cost is quickly recovered. | 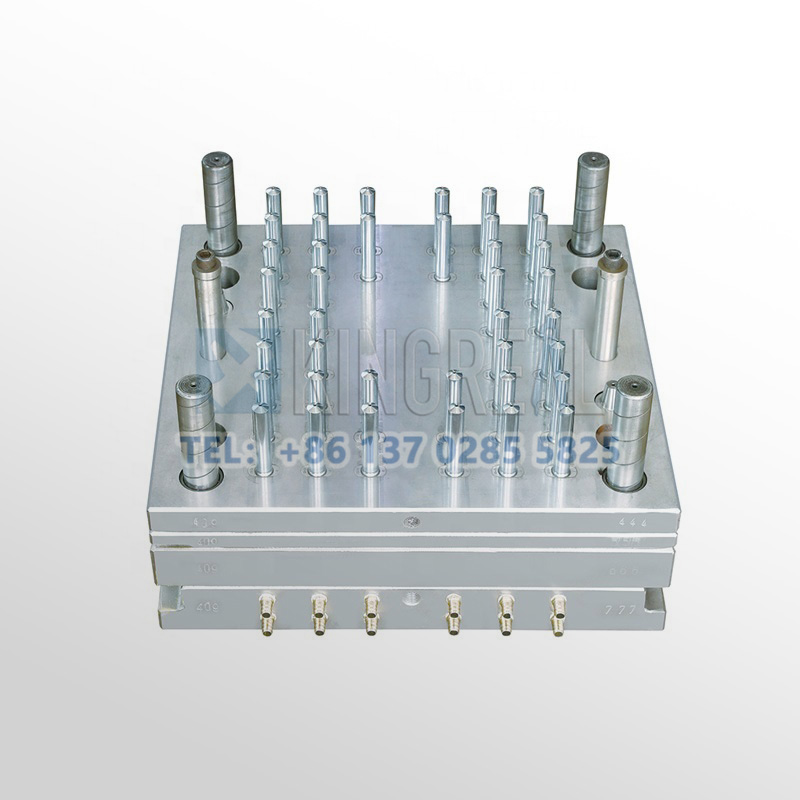 |
4) Customized medical device injection molds Based on the use case and particular customer needs, KRMOLD may create unique medical device plastic injection mold manufacturing solutions. With wall thicknesses between 0.5 mm and 6 mm, KRMOLD medical device injection molds may create plastic pieces weighing anything from a few grams to several pounds. For smaller medical device components, KRMOLD engineers utilize micro injection molding technology.
5) Repeatable medical device plastic injection molds KRMOLD medical device plastic injection molds implement strict process control, automatically monitoring process parameters such as temperature, pressure, and cycle time to ensure that every part meets specifications. KRMOLD offers inspection methods that allow easy visual or automated detection of part defects, ensuring product quality.
6) Standard-compliant medical device plastic injection molds KRMOLD guarantees that medical device plastic injection molds generated meet regulatory standards of the customer's area by using medical device standards. demands in areas including the US FDA, the European CE Mark, and the Japanese PAL. In terms of materials, manufacturing methods, sterilizing techniques, and bio-compatibility testing, KRMOLD medical device injection molds adhere to medical device requirements and quality standards. |  |
Common Plastic Injection Molding Techniques for Medical Device Injection Mold
——
1) Gas-assisted injection molding
Gas-assisted injection molding solves the problems with unequal drying or curing of thicker components seen in typical molding. Nitrogen is flowed through channels made into the medical device injection mold, therefore giving the resin the necessary pressure to produce smooth, structurally sound components.
Usually used to make a tight, leak-proof joint, insert molding lets a second piece be cast on top of a pre-molded one. Putting an already present component into a medical device injection mold and then injecting melted plastic creates a robust molecular or mechanical connection. This technique is widely used in the production of various medical devices.
Overmolding coats a polymer onto a substrate to create a single, integrated component, hence doing away with the requirement of assembly. This technique is perfect for high-volume manufacturing and is sometimes used to produce ergonomic surgical instrument handles.
4) Liquid silicone rubber injection molding
Liquid silicone rubber injection molding is suitable for the production of medical devices with high hygiene requirements, such as tubing and respiratory masks. This technique promises a dust-free and moisture-free production environment and produces a chemically resistant, rubber-like substance appropriate for safe implantation.
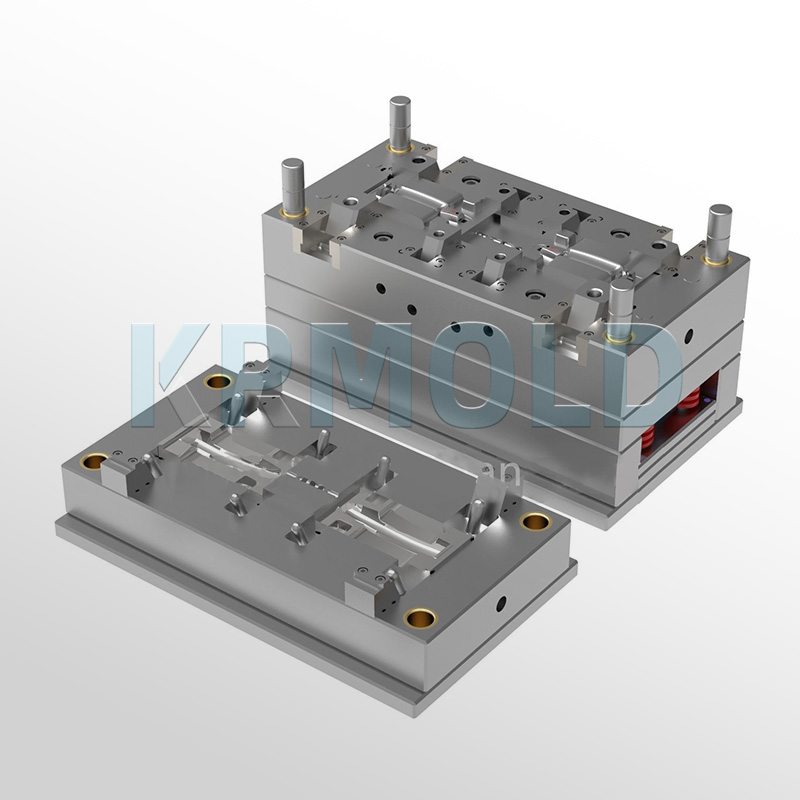
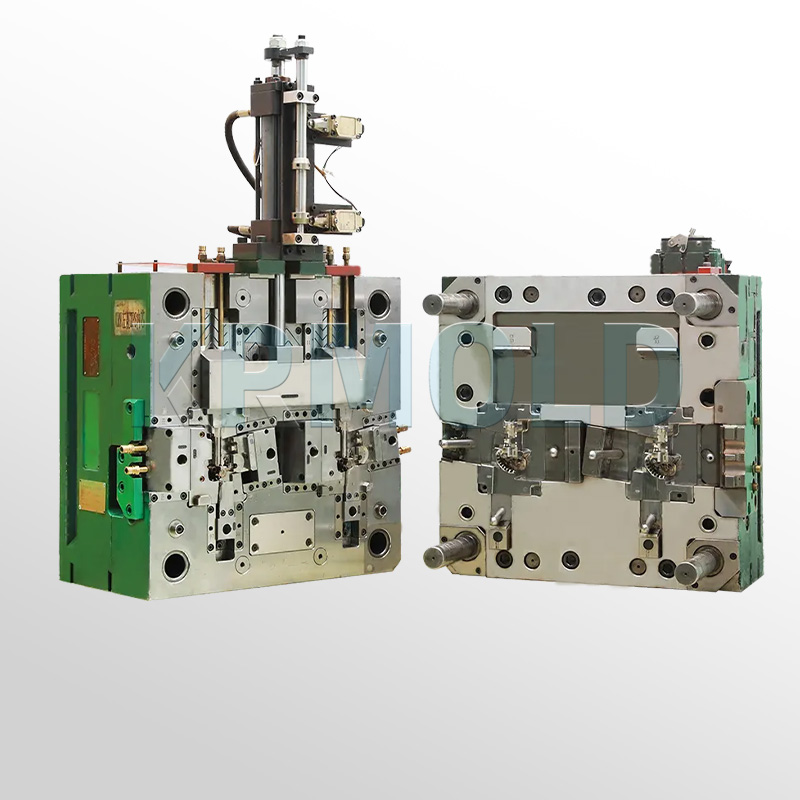

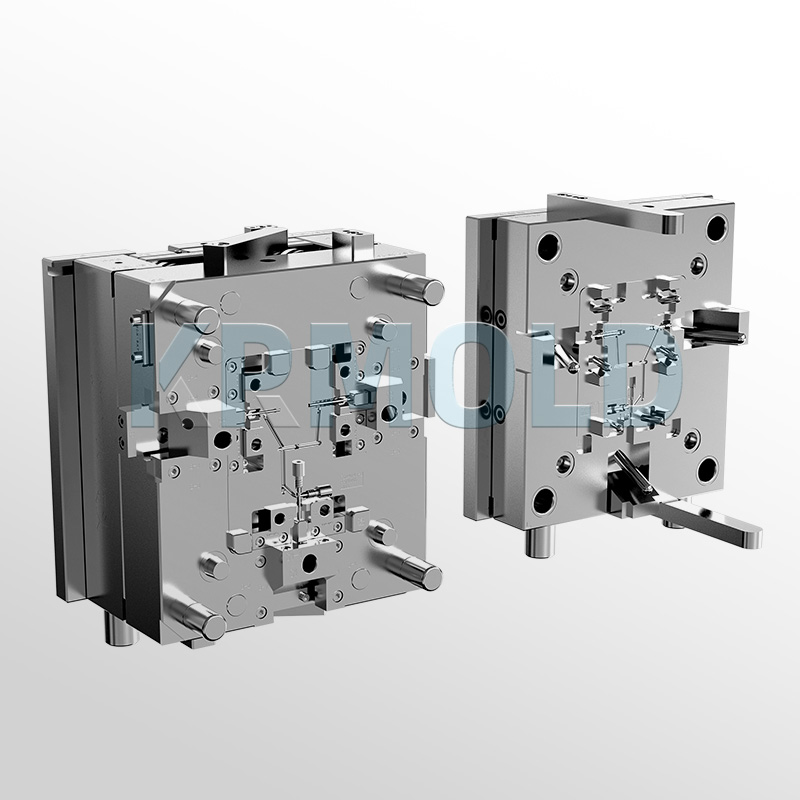
Common Plastic Materials Used in Medical Device Injection Molding
——
| Resin | Wall Thickness (mm) |
| Acetal (POM) | 0.76 - 3.05 |
| Acrylic (PMMA) | 0.025 - 0.150 |
| Acrylonitrile Butadiene Styrene (ABS) | 1.14 - 3.56 |
| Nylon (PA) | 0.76 - 2.92 |
| Polybutylene Terephthalate (PBT) | 2.032 - 6.350 |
| Polycarbonate (PC) | 0.040 - 0.150 |
| Polyetheretherketone (PEEK) | 0.020 - 0.200 |
| Polyetherimide (PEI) | 0.080 - 0.120 |
| Polyethylene (PE) | 0.030 - 0.200 |
| Polyphenylsulfone (PPSU) | 0.030 - 0.250 |
| Polypropylene (PP) | 0.040 - 0.150 |
| Polystyrene (PS) | 0.025 - 0.125 |
| Thermoplastic Elastomer (TPE) | 0.025 - 0.125 |
| Thermoplastic Polyurethane (TPU) | 0.025 - 0.125 |
Applications of Medical Device Plastic Injection Mold
——
-Medical Device Housings and Components: Housings, panels, buttons, connectors, etc.
-Syringe and Infusion Set Parts: Syringe plungers, syringe barrels, infusion set connectors, valves, etc.
-Respiratory Devices: Plastic parts for medical devices such as respirators and oxygen masks.
-Medical Containers: Plastic containers, bottles, and pharmaceutical packaging for medical applications.
-Dental Devices: Plastic parts for dental cups, dental impressions, and dental braces. Surgical Tools: Handles, grips, and other components of surgical tools and instruments.


Medical Device Plastic Injection Molding Process
——
-Raw Material Preparation: Prepare plastic raw materials that meet medical industry standards based on medical product design requirements.
-Medical Device Plastic Injection Mold Design: Design and manufacture medical device injection molds for medical devices based on product shape and size requirements.
-Injection Molding Machine Operation: Place plastic raw materials into the injection molding machine, heat and pressurize them to melt, and then inject them into the medical device injection mold.
-Cooling and Solidification: The plastic cools and solidifies in the medical device plastic injection mold, forming the desired product shape while ensuring physical properties and dimensional accuracy.
-Medical Device Plastic Injection Mold Opening and Part Removal: Open the medical device plastic injection mold and remove the solidified plastic product, ensuring it is free of damage or deformation.

Specify the type of plastic (e.g. PP, ABS) and post-processing requirements (e.g. spraying, silk-screen printing), and provide 2D or 3D plastic part drawings should be provided. At the same time, provide the production volume, appearance requirements, tolerance standards, etc.
Generally speaking, our engineers will start to prepare the quotation immediately after the customer provides the complete production requirements. Usually it takes about 1-3 days.
The lead time for regular injection molds is usually 30-60 days, and may be longer for complex molds. For example, the typical lead time for liquid silicone molds is around 60 days, covering design, manufacturing, mold testing, etc.
High-precision processing technology: High-precision equipment such as CNC machining centers (CNC) and electric discharge machining (EDM) are used to optimize the design process in combination with CAD/CAM software. Quality control: Inspection of key dimensions of the mold by Coordinate Measuring Machine (CMM) and verification of multiple sample batches during the trial molding stage. Material Selection: Use die steel with high wear resistance (e.g. H13, S136) and surface treatment (e.g. nitriding, chrome plating) for die nuts to extend the life.
After every 50,000 molds, check the guide pillar, ejector pin and other wear parts, and clean up the residual plastic and rust on the mold surface. Use high temperature grease for sliding parts (e.g. tilt top, slider) to reduce friction loss. Ensure that the water circuit is smooth and the temperature difference is ≤5℃ to avoid cracking of the mold due to thermal stress.
Mould cost of materials accounted for about 30-40% (such as 1 ton of P20 steel price of about 20,000 yuan), processing costs accounted for more than 50% (CNC labor hourly rate of about 80-150 yuan / hour). Small batch production can choose aluminum mold or simplify the structural design; more than 100,000 pieces is recommended to use carbide inserts to enhance life!
Mould injection products need to fully meet the design requirements (such as size, appearance), and can be continuous and stable production. Mold marking, inspection reports (such as material hardness test) and engineering drawings should be complete.
Mould steel (such as S136H, NAK80 and other imported materials cost more) and the type of mold embryo (aluminum mold short-term cost is low but short life) directly affect the cost, the use of CAD/CAE/CAM design technology, hot runner system, etc. will increase the upfront investment, but can enhance the long-term benefits (such as reducing the sprues, increase production capacity).